Cryo-EM at the Forefront of AI Drug Discovery: Exploring Deep Chemical Space
How Model Medicines' GALILEO™ Platform and its Constellation™ model are Transforming AI-Driven Drug Discovery
A profusion of biological data, both in literature and database formats, covering numerous aspects of human biology is coercing a revolution of data driven methodologies into the field of pharmaceutical drug development.
Despite the abundance of biological data, significant challenges remain to obtain datasets both clean enough and large enough to train powerful, modern deep learning algorithms for Artificial Intelligence (AI) based Drug Discovery.
Cryogenic Electron Microscopy (Cryo-EM) is a cutting edge method, which enables the creation of large, clean datasets that if properly utilized have the potential to unlock the future of AI based Drug Discovery. Data acquisition rates on the order of 1 PB/day are widely expected in the near future.
In this Paper, we present the processing of Cryo-EM raw data to train our Constellation™ model, an AI model created to discover chemistry that enables small molecules to bind to protein targets and learns from naturally occurring protein-protein interactions.
Specifically, we demonstrate proprietary state of the art image processing to reconstruct protein structures from 2-dimensional microscopy data, and exemplify our vision and capability to leverage cutting-edge Cryo-EM data to train Constellation™.
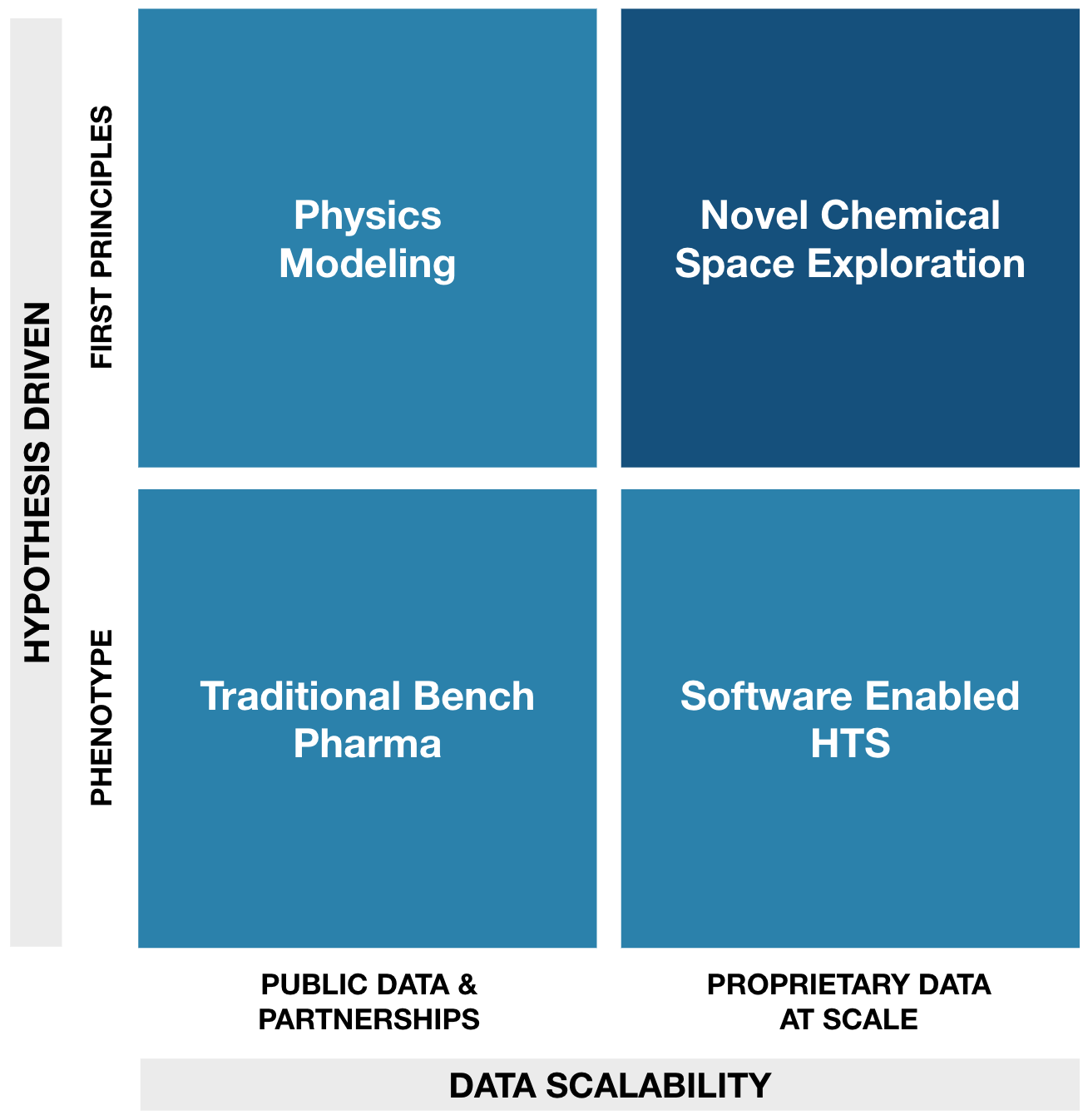
Traditional approaches to drug discovery have explored only a small percentage of available chemical space. The next generation of novel therapeutics will come from the unexplored chemical spaces. To explore novel chemical space, AI platforms like GALILEO™ require scalable data and algorithms able to deduct meaning from first principles, see Figure 1. Cryo-EM data and the Constellation™ model provide Model Medicines a pathway to accomplish both.
AUTHOR
Bastiaan Bergman, Ph.D
VP of Engineering, Model Medicines